Irema becomes MDR compliant (Medical Devices Regulation (2017/745/EU)) more than a year in advance of its coming into effect.
 Irema is a byword for quality and compliance in the manufacture of medical face masks for numerous OEM clients and under the Facemate brand for over 35 years. Under the watchful eye of our Quality Control & Regulatory Affairs department, our industry leading face masks manufactured in our Limerick facility are fully compliant with MDR (Medical Devices Regulation (2017/745/EU)) in advance of its coming into effect in May 2020. (Due to the pandemic, this was pushed back to May 2021).
Irema is a byword for quality and compliance in the manufacture of medical face masks for numerous OEM clients and under the Facemate brand for over 35 years. Under the watchful eye of our Quality Control & Regulatory Affairs department, our industry leading face masks manufactured in our Limerick facility are fully compliant with MDR (Medical Devices Regulation (2017/745/EU)) in advance of its coming into effect in May 2020. (Due to the pandemic, this was pushed back to May 2021).
The new regulation, known as ‘the MDR’, brings EU legislation into line with technical advances, changes in medical science, and progress in law making. The regulation creates a robust, transparent and sustainable regulatory framework across the EU which improves clinical safety and creates fair market access for manufacturers.
Speaking on the introduction of the MDR, Andrew Dawson, Quality & Regulatory Affairs Manager explained:
“Irema has a long history of technical and procedural excellence in the field of regulatory compliance and this new regulation brings us the opportunity to again come to market with a demonstrably safe and effective product ensuring protection for healthcare workers everywhere.”
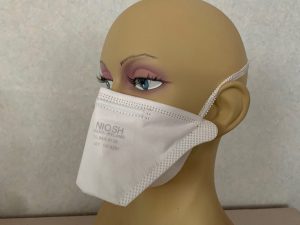
Irema Ireland’s range of MDR compliant respirator face masks is available in N95, FFP2 and FFP3 classifications. The surgical face masks have a Bacterial Filtration Efficiency greater than 98% and come in standard and speciality formats – all of which comply fully with MDR. Irema offers full OEM, private labelling and ‘Facemate’ distribution opportunities for our medical mask product range.
Surgical masks Respirator masks
Back to Blog

